All Takeda is trying to do with its billions-of-dollars worth of licensing deals in gene therapy is cure rare disease. Simple, right?
Madhu Natarajan, head of Takeda’s Rare Disease Drug Discovery unit, knows that to many, cure is a “four-letter word.”
“It's very hard to demonstrate a cure, but my sentiment is that should not prevent you from aspiring towards building a cure,” Natarajan, who came into Takeda through the 2019 Shire acquisition, said in an interview with Fierce Biotech.
This drive for a cure is all in how Takeda defines the word. If a patient can take a therapy—whether once or for the rest of their lives—and achieve a relatively normal state of health without any new symptoms developing, that is a functional cure to Natarajan. And that’s what Takeda is hoping to achieve with a recent spate of deals in gene therapy.
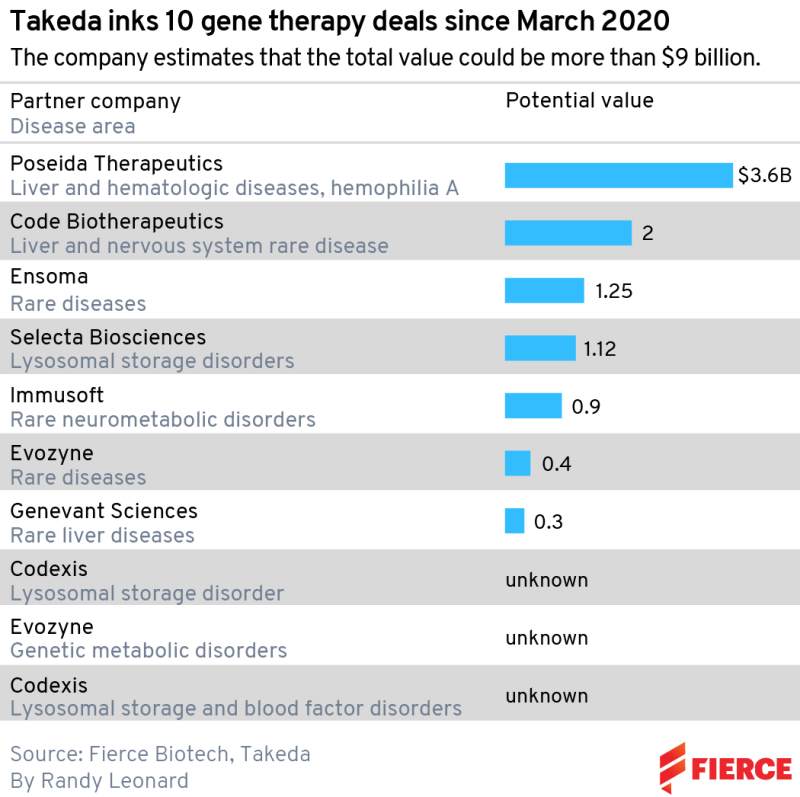
The most recent was a $400 million biobucks collaboration with Evozyne in rare diseases signed in early April. This followed an earlier tie-up between the two companies, signed in January 2021, on genetic disorders and lysosomal storage diseases, which are inherited metabolic disorders that cause certain substances to build up in the body and cause problems. But that was a small agreement compared to Takeda’s other recent moves: $2 billion in milestones for Code Bio; $3.6 billion including $45 million upfront for Poseida Therapeutics; and $1.12 billion for Selecta Biosciences.
While these seems like a lot of disparate moves, Natarajan said each company lends its piece to the larger gene therapy puzzle that Takeda is trying to assemble.
“We will stitch together an ensemble of technologies, which cumulatively will bring about this best-in-class gene therapy,” he said.
Natarajan said the project is aimed at lysosomal storage diseases, hematology and metabolic disorders, focusing on unmet medical needs. Right now, the particular indications that will move into the clinic are yet to be determined.
The goal is not necessarily to create one-and-done therapies, which have been all the rage in gene therapy lately.
"Play nice"
Natarajan pegs the start of his puzzle back to March 2020, when Takeda announced a deal with protein engineering company Codexis. No financial details were disclosed, but the companies agreed to work on up to seven targets in lysosomal storage disorders and blood factor deficiencies. The deal was extended, again with no dollar amounts revealed, in June 2021. Other companies involved include Ensoma, Immusoft and Genevant Sciences.
The overarching goal has been to secure the best possible technology for each piece needed to build a gene therapy, which essentially replace or add a gene to fix an underlying medical problem. The first piece is to build a better transgene, which is the genetic material to be delivered. Then, Takeda is working on the gene cassette that holds the gene plus a DNA sequence called promoter enhancement technology that helps the body understand and express the new gene. The final element is the delivery technology that packages the whole thing up to be administered.
“I would love to say that everything works in a carefully orchestrated plan,” Natarajan said. “But suffice to say that we had an initial sequence of events which allowed us to get started. And then as we expanded, this is where the learnings came in.”
Takeda now thinks it has a collection of companies that can build a best-in-class gene therapy program. Many biotechs claim to be the best at one technology or the other. But Natarajan said another important factor when going through the dealmaking process was that the leadership teams, scientists and everyone involved could get along well with others.
“Everyone believes that they have it in them to be a one-stop shop to do everything, or that their technology is good enough for them to build a gene therapy,” Natarajan said. “We probed them to see how many of them see the big picture, which is the vision that, are you willing to be a partner in a larger consortium?”
Finding the perfect biotechs to fit those criteria was not easy; as Natarajan said, “that calls for a special breed of companies.” But Takeda’s internal researchers, who will oversee the project from the top down, believe they have found their crew.
“They checked off every box on the technology component, as well as the collegial component if you will, in terms of how they are willing to play nice,” he said.
So, is the team complete?
“Until such time that I am able to show phase 1 data, where things are proof positive, I will always have a wish list,” Natarajan said.
Right now, the group has the components to do just that—with a read-out “a lot closer” than five years away, maybe one to two years from now.
But as Natarajan and his teams learn more, they may consider adding additional deals, targets or companies. He said the flurry of deal announcements over the past 12 to 18 months was simply an indication that they were in the build stage; now, the project is moving into the growth phase.
“As we get closer to a steady state, I don't anticipate a large bolus of deals. But I do anticipate that based on the learnings that we have with some of these deals, we may choose to augment or we may choose to replace,” Natarajan said.
The biotechs also get the benefit of working with a well-established pharmaceutical partner with the clinical trial experience and expertise to move through the cumbersome, laborious drug development process.
Natarajan credits his internal researchers for being able to work with such a large group of outside scientists toward one common goal. His team is designing everything, from what experiments get done to which programs advance.
“If I did not have the internal expertise that I have, pulling off this model that we have set up would be absolute madness. I just couldn't do it,” Natarajan said. “Takeda internal research is just as strong a collaboration partner and honestly is the glue that keeps all of these collaboration pieces together.”