The FDA has granted a groundbreaking approval to a fluorescent light system that illuminates leftover breast cancer tissue in real-time during surgery—to help ensure a thorough removal of the tumor and avoid the need for follow-up procedures.
Lumicell’s approach starts with an injectable imaging agent, Lumisight (pegulicianine)—which is inactive at first but begins to glow when it interacts with particular enzymes found around tumor cells.
At the same time, the company’s computerized, handheld imaging probe scans the inside of the surgical cavity created during a breast-conserving lumpectomy and logs any signs of residual cancer hidden among the healthy cells.
Lumisight was approved via the FDA’s pharmaceutical review pathway—after receiving a positive recommendation from an independent advisory committee in early March—while Lumicell’s Direct Visualization System hardware took a parallel track through the agency’s device center.
“We are immensely proud of the dual approval of Lumisight and Lumicell DVS—we believe this is the first drug-device combination product approved in over a decade to have followed both of the FDA’s most stringent NDA and PMA review processes,” Lumicell’s president and chief operating officer Howard Hechler said in a statement. “With the FDA’s approval, LumiSystem is now the first and only imaging combination product capable of detecting cancerous tissue where it matters most, inside the breast cavity.”
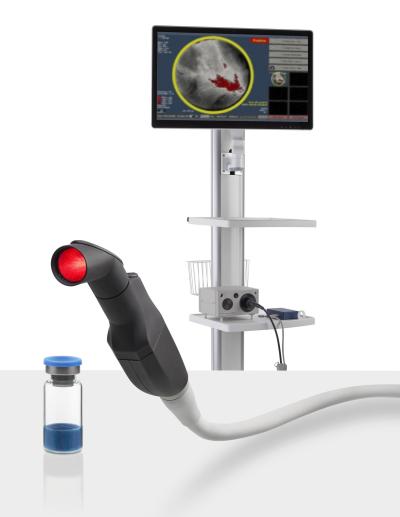
According to the company, about one-third of women who undergo the removal of a breast tumor have to return to the operating room for another surgery. Meanwhile, about two-thirds of those follow-up procedures end up not finding any residual cancer, leaving clinicians to wonder if previous assessments were wrong or if the cancer was simply missed a second time.
Lumicell said its system—which previously received both the FDA’s pharmaceutical fast track and breakthrough device designations—can provide 84% diagnostic accuracy.
In a clinical trial of about 400 participants undergoing breast cancer surgery, follow-up procedures were avoided in 9 of 62 patients that had positive tumor margins. At the same time, 27 of 357 patients in the LumiSystem arm had extra tumor tissue removed after a standard lumpectomy, including 22 who were initially ascribed negative margins.
Administration of the Lumisight imaging agent was halted due to side effects in six patients; two patients developed serious adverse events related to pegulicianine. The study’s results were published in April 2023 in the journal NEJM Evidence.
Lumicell is also studying its combination imaging approach in gastrointestinal tumors, sarcomas and peritoneal metastases—the last of which can be difficult to detect with a CT, PET or MRI scan—as well as in brain and prostate cancers.
Last October, Lumicell co-founder Moungi Bawendi shared the 2023 Nobel Prize in chemistry for his research in the discovery and synthesis of quantum dots—nano-sized particles used in optical electronics ranging from solar cells to LED displays to lasers, as well as biomedical imaging.
The company’s fluorescent visualization system was developed in Bawendi’s lab at the Massachusetts Institute of Technology, where he serves as the Lester Wolfe Professor of Chemistry. According to Lumicell, Bawendi’s expertise in optics and tissue imaging helped guide the design of the device, while his work on the physio-chemical properties of fluorescent molecules led to the imaging agent.
A separate company developing a similar injectable approach for lung and ovarian cancers, On Target Laboratories, raised $30 million in a series C round last November to help commercialize its Cytalux (pafolacianine) fluorescent agent and near-infrared imaging device.