An Apple Watch app from H2o Therapeutics certainly seems to hold water as a Parkinson’s disease monitoring system, as the FDA handed down 510(k) clearance for the technology last week.
The Turkish company announced the agency’s decision Monday. It will allow the approximately 500,000 people in the U.S. diagnosed with Parkinson’s to use the Parky app to help track tremors, dyskinesia and other symptoms of the disease.
Once Parky is downloaded onto a user’s iPhone and Apple Watch, its machine learning algorithms continuously analyze the smartwatch’s movement data to automatically pick up on Parkinson’s-related symptoms. Users can also manually input additional symptoms into the app, set up medicine reminders and access daily exercise programs and educational information about Parkinson’s.
The app can also guide users through certain medical episodes: For example, if they experience “freezing,” a common symptom in which patients report feeling unable to move for several seconds or minutes, a caregiver can activate Parky’s auditory cues—including a playlist of metronome sounds and songs set to a slow walking rhythm—to help trigger movement.
All of the data gathered by the app are compiled into daily, weekly and monthly reports that patients can choose to share directly with selected caregivers and healthcare providers.
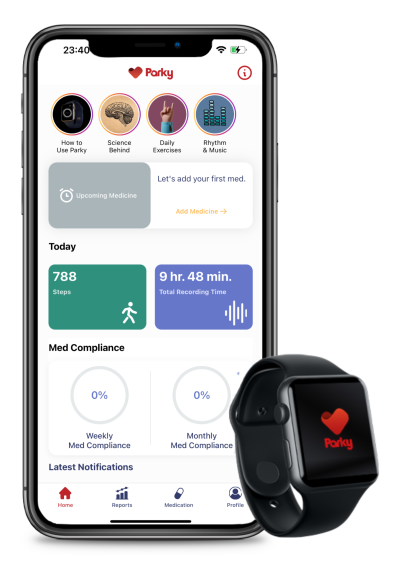
H2o’s technology relies on an API that Apple embedded into its eponymous wearable for the express purpose of monitoring movement disorders.
A study published in the journal Science Translational Medicine last year put the Parky app to the test, tasking it with tracking fluctuations in resting tremor and dyskinesia, the uncontrolled, involuntary muscle movements common among Parkinson’s patients.
In the study, the app’s measurements matched closely with clinicians’ own ratings of tremor severity and dyskinesia while participants performed tasks in the clinic. Additionally, the technology was able to capture post-treatment symptom changes that matched up with clinicians’ expectations in 94% of users, and in the remaining 6% where tracked symptoms showed less-than-ideal results of a treatment strategy, the app’s data helped guide clinicians in improving that strategy.
Those results proved the technology’s potential “as a tool to support patient-clinician communication, medication titration and clinical trial design,” the study’s authors wrote.
With FDA clearance for Parky secured, H2o Therapeutics is diving headfirst into even deeper waters, with plans to submit two more mobile apps for regulatory review next year, the company said in Monday’s announcement.
Its website lists Foggy, a digital therapeutic that will use augmented reality and artificial intelligence to help treat certain neurodegenerative diseases, and Covie, an app for smartwatches and smartphones to catch the onset of COVID-19 as early as possible, as additional technologies currently in development.