For years, Medtronic has trailed behind Dexcom and Abbott in the continuous glucose monitor market. Its newest entry in the space, however, may give its competitors a run for their money.
The slimmed-down Simplera CGM has earned CE mark clearance, Medtronic announced Thursday, setting it up for a launch in Europe in the near future.
The sensor is half the size of Medtronic’s last CGM, the Guardian 4, while also being easier to apply than its predecessor—via a two-step insertion process, using the included applicator tool—and is completely disposable. Plus, unlike the Guardian 4 sensor, the Simplera device adheres to the back of a user’s arm without requiring any additional tape or adhesive to keep it in place.
The flatter, simplified sensor more closely resembles Dexcom and Abbott’s offerings, though Simplera’s seven-day wear period is the shortest of the three compared to the Dexcom G7’s 10-day span and Abbott FreeStyle Libre 3’s 14-day period.
The CGM’s European rollout will happen in phases, according to Medtronic, with the phased launch kicking off at the European Association for the Study of Diabetes annual meeting in Germany early next month.
The CE mark approval allows the Simplera sensor to be used alongside Medtronic’s InPen, a reusable insulin pen that uses Bluetooth to transmit dosage data to a mobile app. In addition to showing the dose history, as well as blood sugar readings from a connected CGM, the app features a dose calculator that analyzes those glucose readings to suggest optimal meal and correction doses of insulin.
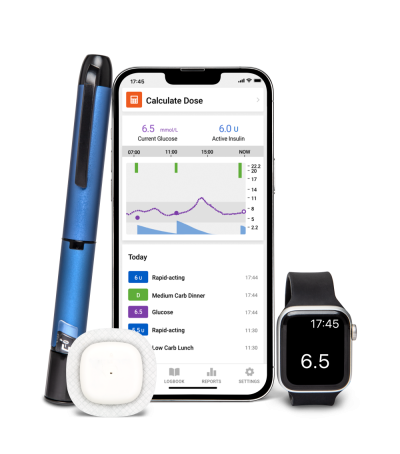
The app also sends out alerts when it picks up on rising glucose readings from the CGM or detects a possible missed mealtime dose, urging users to take a dose of insulin to return to healthy levels.
The Simplera CGM, InPen and personalized dose-suggesting app are all part of what Medtronic has dubbed its Smart MDI ecosystem of tools to help people who take multiple daily injections better manage their diabetes.
“Despite the rapid adoption of CGM over the past decade, less than 30% of individuals on MDI therapy using a CGM achieve glycemic targets—highlighting a significant unmet need. We’re excited to help more people to reach their goals with our advanced algorithm in InPen powered by our smallest and most comfortable CGM to-date,” Que Dallara, executive vice president and president of Medtronic Diabetes, said in the announcement.
The Simplera sensor was cleared in Europe for use by adults and children aged 2 and up, and the connected app is compatible with both iOS and Android devices.
Simplera, which ushers in a new generation of Medtronic’s glucose monitoring devices, has been in the works for several years. Medtronic first revealed that a pivotal trial of the technology—known at the time as Project Synergy—was underway in 2020 before submitting it for CE mark approval last year.
The company is still awaiting FDA review, having only applied for Simplera’s U.S. clearance this spring after locking down the agency’s approval for the MiniMed 780G insulin pump and the Guardian 4 sensor in April.
Future iterations of the technology would integrate with the automated insulin delivery (AID) algorithm currently used by the MiniMed pumps and Guardian CGMs. The combination of the Simplera tech and AID algorithm is currently under CE mark review, according to Medtronic’s announcement, but isn’t yet available in Europe or the U.S.